Innovative Technologies for Removing Biofilms: What’s on the Horizon?
Biofilms are clusters of microorganisms, including bacteria, fungi, and other microbes, that adhere to surfaces and produce a protective matrix made up of extracellular polymeric substances (EPS). These biofilms can form on almost any surface—ranging from medical devices, pipes, and industrial machinery to even natural environments like rivers and oceans. In many cases, biofilms pose significant challenges in healthcare, industry, and environmental management due to their resilience against conventional cleaning methods, antibiotics, and antimicrobial agents.
Removing or disrupting biofilms has therefore become a critical area of research, with significant advancements in understanding their structure, behavior, and mechanisms of resistance. As we explore more innovative approaches, new technologies are emerging that promise more effective, targeted, and sustainable solutions for biofilm removal. In this blog, we’ll take a closer look at some of the most promising innovative technologies for biofilm removal and what the future holds for this vital area of research.
Enzymatic Biofilm Disruption:
Enzyme-based technologies have shown great promise in biofilm removal, leveraging the natural processes of enzymes to break down the extracellular matrix (the EPS) that holds biofilms together. These enzymes target specific components of the biofilm, such as polysaccharides, proteins, and lipids, effectively degrading the biofilm structure and making it easier to clear away.
- DispersinB, a type of enzyme that targets the polysaccharides in biofilms, is one example. It works by breaking down the sticky extracellular matrix, reducing biofilm adhesion and making the microorganisms more susceptible to treatment.
- Proteases and lipases are other enzymes that have shown potential in breaking down the biofilm’s protein and lipid components, weakening its structure.
Recent developments in bioengineering have also led to the creation of genetically engineered enzymes that are more efficient at targeting biofilm-specific components, enhancing the effectiveness of biofilm disruption. These enzymes could be used in a wide range of applications, from cleaning medical devices to preventing industrial pipe blockages.
Benefits:
- Selective action: Enzymes can be tailored to target specific biofilm components, minimizing collateral damage to surrounding tissue or equipment.
- Biodegradable: Enzymatic solutions are often biodegradable and eco-friendly, making them a sustainable alternative to chemical cleaners.
Ultrasound and Acoustic Waves:
Ultrasound and acoustic waves are emerging as powerful tools for biofilm removal. High-frequency sound waves are used to generate acoustic cavitation, a process where tiny bubbles form and collapse within a liquid medium. The implosion of these bubbles creates localized shear forces that disrupt the biofilm structure, breaking the biofilm apart.
Ultrasound is particularly useful because it can penetrate complex biofilm structures and reach deeper layers without the need for physical scraping or chemical treatments. By applying focused ultrasound waves, researchers can remove biofilms from hard-to-reach places, such as the interior surfaces of medical implants or industrial pipes.
Benefits:
- Non-invasive: Ultrasound can be applied without direct contact, making it ideal for delicate surfaces or medical devices.
- Targeted: Focused ultrasound can be precisely controlled to target biofilms without affecting the surrounding tissue or environment.
- Versatile: This technique can be used in various industries, from healthcare (for cleaning catheters and surgical instruments) to environmental management (e.g., cleaning water pipes).
Photodynamic Therapy (PDT):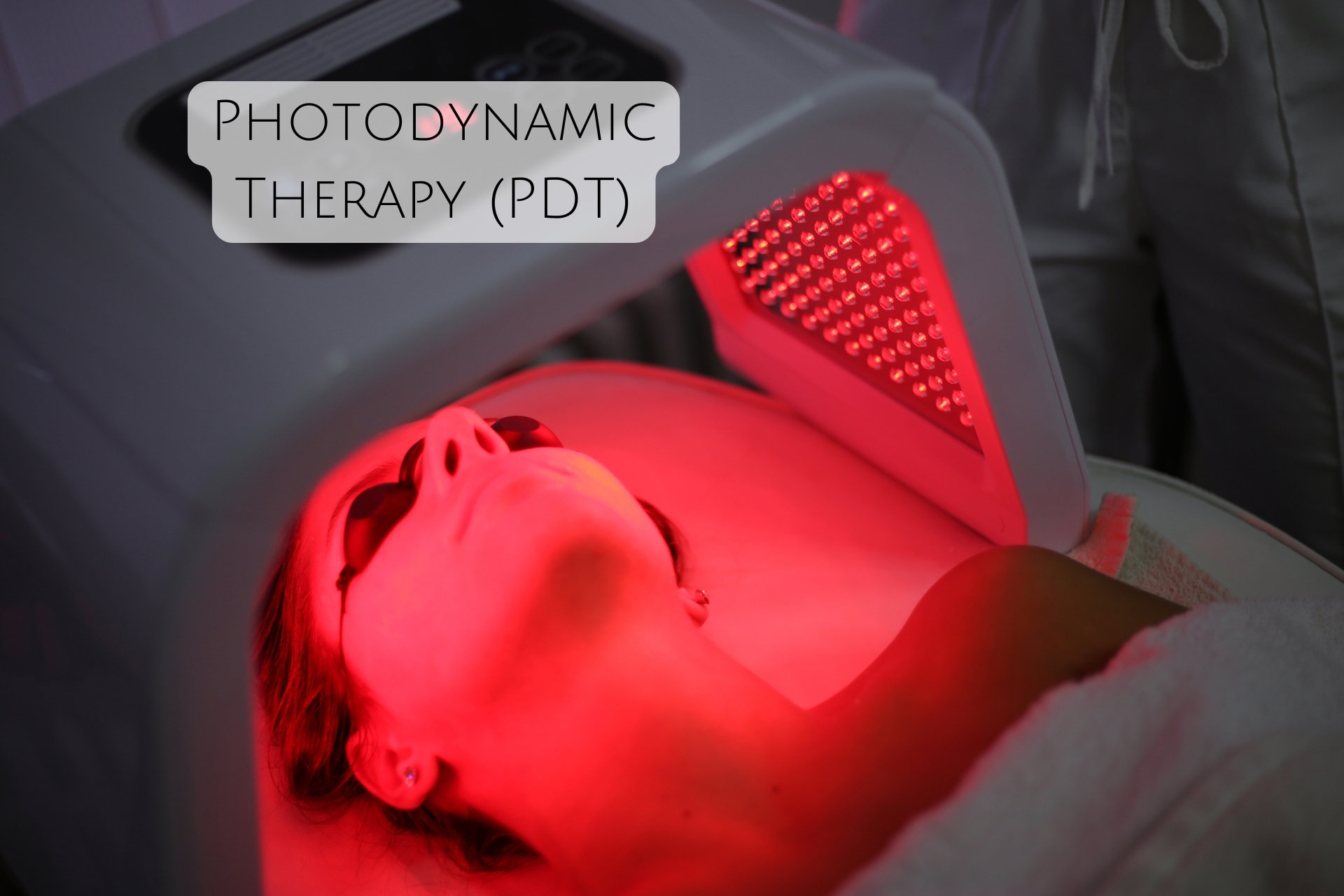
Photodynamic therapy (PDT) is an innovative approach that uses light-activated compounds (photosensitizers) to treat biofilms. These photosensitizers are introduced to the biofilm and activated by light, leading to the production of reactive oxygen species (ROS) that damage microbial cells and the extracellular matrix.
This technology is especially promising in medical applications, where PDT can be used to target biofilms on wounds, implants, and surgical devices. PDT is effective against both bacterial and fungal biofilms and offers a targeted, non-invasive alternative to traditional antibiotics.
Recent advancements in PDT have led to the development of nanomaterials that can more effectively deliver photosensitizers to biofilms and enhance the ROS generation, improving the overall treatment outcome.
Benefits:
- Targeted action: PDT can be precisely controlled and targeted to specific biofilm areas, minimizing damage to surrounding tissue.
- Antibiotic-sparing: PDT provides an effective alternative to antibiotics, which is especially important in the face of rising antibiotic resistance.
Challenges:
- Light penetration: Deep biofilms may be difficult to treat with PDT due to limited light penetration, although advancements in nanotechnology are working to overcome this limitation.
- Photosensitizer toxicity: Some photosensitizers may have toxic side effects, which need to be carefully considered in clinical applications.
Bacteriophage Therapy: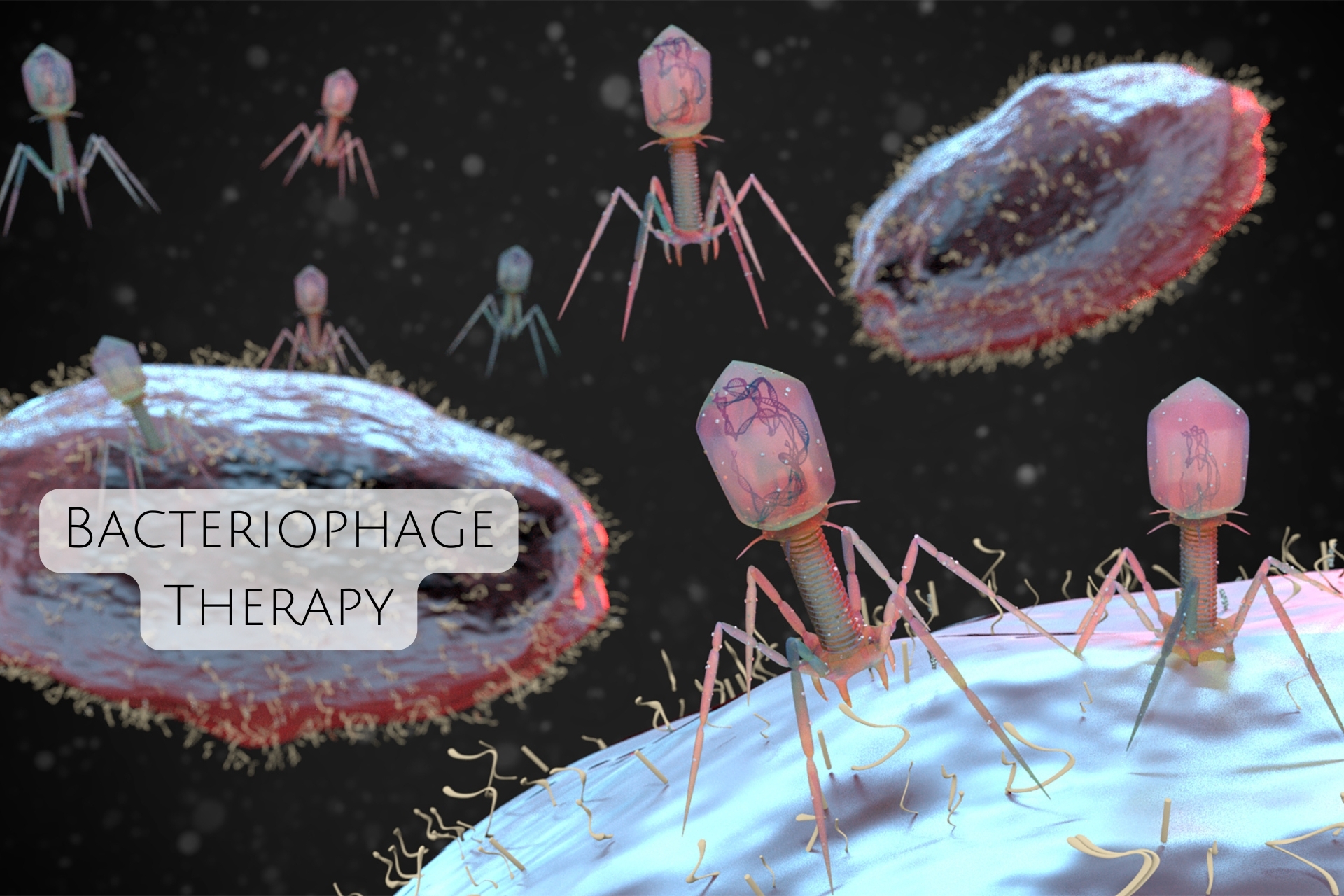
Bacteriophages are viruses that specifically target and infect bacteria. When used to treat biofilm-associated infections, bacteriophage therapy involves introducing phages that selectively infect and kill the bacteria within the biofilm, without harming human cells or beneficial microbes.
Bacteriophages have shown great promise in combating antibiotic-resistant infections, particularly those associated with biofilms. By using bacteriophages, it’s possible to break down biofilms and reduce the bacterial load, potentially offering a more precise and effective solution to biofilm-related infections.
Benefits:
- Selective killing: Bacteriophages specifically target bacterial cells, reducing the risk of harm to surrounding tissue.
- Antibiotic alternative: Bacteriophages provide an option for treating antibiotic-resistant biofilm infections.
Challenges:
- Phage resistance: Just like bacteria can develop resistance to antibiotics, they can also evolve resistance to bacteriophages, which may limit long-term effectiveness.
Electrokinetic and Electric Field-Based Approaches:
Electrokinetic techniques apply electric fields to move charged particles within the biofilm. This can disrupt the biofilm’s structure, promote the release of microbial cells, or even enhance the penetration of antimicrobial agents into the biofilm matrix.
In electric field-based biofilm removal, electric currents are used to weaken the biofilm’s protective matrix, allowing it to break apart or become more susceptible to treatment with antibiotics or other agents.
Benefits:
- Non-chemical approach: These methods don’t rely on harsh chemicals or antibiotics, making them more sustainable and environmentally friendly.
- Synergistic: When combined with other treatments (e.g., antibiotics or enzymatic treatment), electric fields can enhance the effectiveness of biofilm removal.
Challenges:
- Limited application: Electrokinetic methods are most effective in specific environments, such as in biofilms on surfaces that can be easily exposed to electric fields.
Conclusion: The Future of Biofilm Removal:
The challenge of biofilm removal remains a complex problem across various industries, from healthcare to water treatment. However, the rapid development of innovative technologies such as enzymatic disruption, ultrasound, photodynamic therapy, bacteriophage therapy, and electric field-based approaches is offering promising solutions to tackle biofilms effectively.
As research continues and these technologies are refined, we can expect more efficient, cost-effective, and sustainable methods to emerge, revolutionizing the way we manage biofilm-related issues. With cross-disciplinary advancements, including nanotechnology, synthetic biology, and smart materials, the future of biofilm removal looks increasingly innovative and promising. The goal is not just to remove biofilms but to prevent their formation in the first place—offering long-term benefits for both human health and industrial applications.
Recent Posts
-
The Role of Un-Natured Protein in Enhancing Detoxification
While the human body is well-equipped with a detoxification system—primarily managed by the liver, k
-
The Role of the HOMA Test in Assessing Metabolic Health
In today’s fast-paced world, where health conditions such as Type 2 diabetes, obesity, and metabolic
-
The Role of Omega-3s in Brain Health and Heart Disease Prevention
Omega-3 fatty acids are essential polyunsaturated fats that play a crucial role in maintaining overa





